PPT LAB 3 Enzyme PowerPoint Presentation, free download ID4526880
Moof's Medical Biochemistry Video Course: http://moof-university.thinkific.com/courses/medical-biochemistry-for-usmle-step-1-examFor Related Practice Problem.

The LineweaverBurk plot for the ACE inhibition pattern of purified... Download Scientific Diagram
What Is Lineweaver Burk Plot? A Lineweaver Burk Plot is the graphical representation of the Lineweaver Burk Equation. The plot is used to compare with no inhibitor to identify the effectiveness of the inhibitor. The following describes the Lineweaver Burk plot's components, Substrate Concentration

LineweaverBurk plot of the inhibition of nitric oxide synthase... Download Scientific Diagram
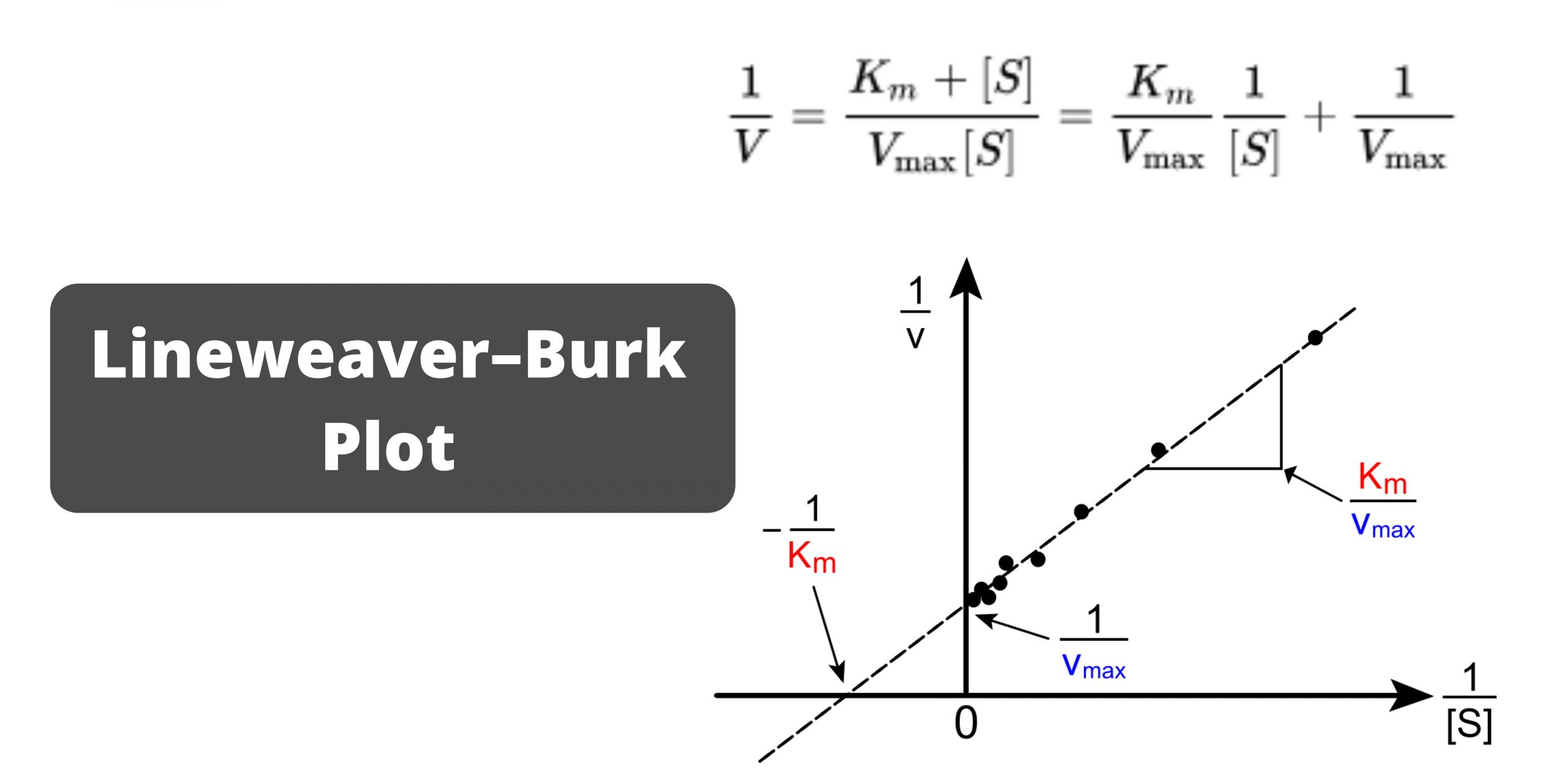
The Lineweaver-Burk plot was widely used to determine important terms in enzyme kinetics, such as \(K_m\) and \(V_{max}\), before the wide availability of powerful computers and non-linear regression software. The y-intercept of such a graph is equivalent to the inverse of \(V_{max}\); the x-intercept of the graph represents \(−1/K_m\)..
Lineweaver Burk plot. The data on Xaxis indicate the 1/substrate while... Download Scientific
Lineweaver Burk plots show that the Vmax was calculated at 9 nmoles per mg per 30 min, or 1.3 nmoles per pineal per 30 min. From: Serotonin and Behavior, 1973 Add to Mendeley About this page Molecular Aspects of Inhibitor Interaction with PDE4 Siegfried B. Christensen,. Theodore J. Torphy, in Phosphodiesterase Inhibitors, 1996

LineweaverBurk Plot Biochemistry, Enzyme Mcat
For this mechanism, Lineweaver-Burk plots at varying A and different fixed values of B give a series of parallel lines. An example of this type of reaction might be low molecular weight protein tyrosine phosphatase against the small substrate p-initrophenylphosphate (A) which binds to the enzyme covalently with the expulsion of the product P.
LineweaverBurk Plot
To determine the V max from a Lineweaver-Burk plot you would: A. Multiply the reciprocal of the x-axis intercept by -1. B. Multiply the reciprocal of the y-axis intercept by -1. C. Take the reciprocal of the x-axis intercept. D. Take the reciprocal of the y-axis intercept.

Lineweaver Burk double reciprocal plots for the determination of Km... Download Scientific
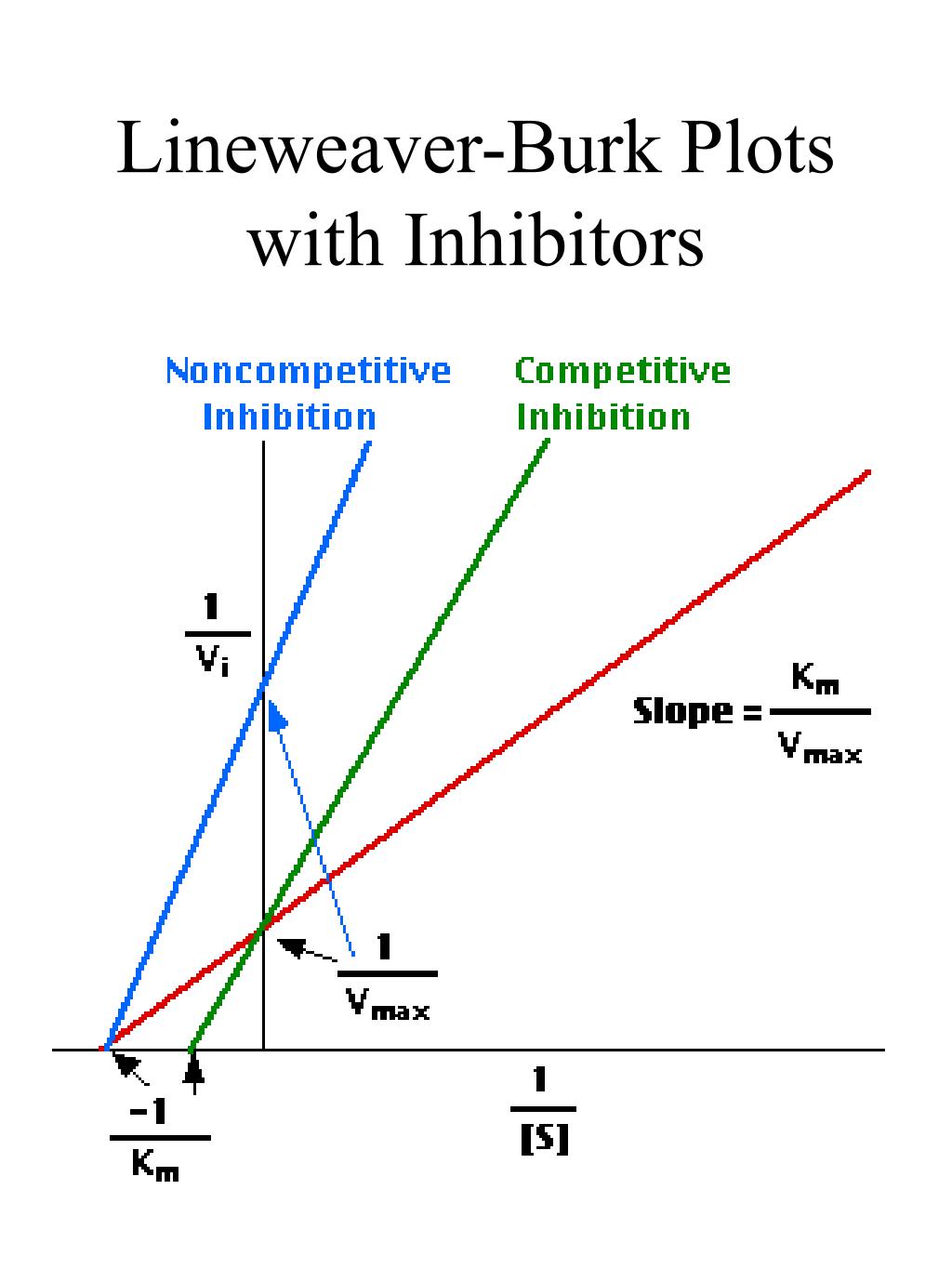
The Lineweaver-Burk plot shows both lines meet the y axis at the same place. In contrast, the following plot shows noncompetitive inhibition. Once again, the regular line is the lower one, whereas the upper line is the inhibited one. The two lines do not share the same y intercept, however. However, they do share the same x intercept.

LineweaverBurk plots for steady state inhibition of AChE (A) and BChE... Download Scientific
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.

Double reciprocal 1/V versus 1/[S] lineweaver burk plot for the... Download Scientific Diagram
The Lineweaver-Burk plot (or double reciprocal plot) is a graphical representation of the Lineweaver-Burk equation of enzyme kinetics, described by Hans Lineweaver and Dean Burk in 1934. This plot is a derivation of the Michaelis-Menten equation and is represented as: Table of Contents
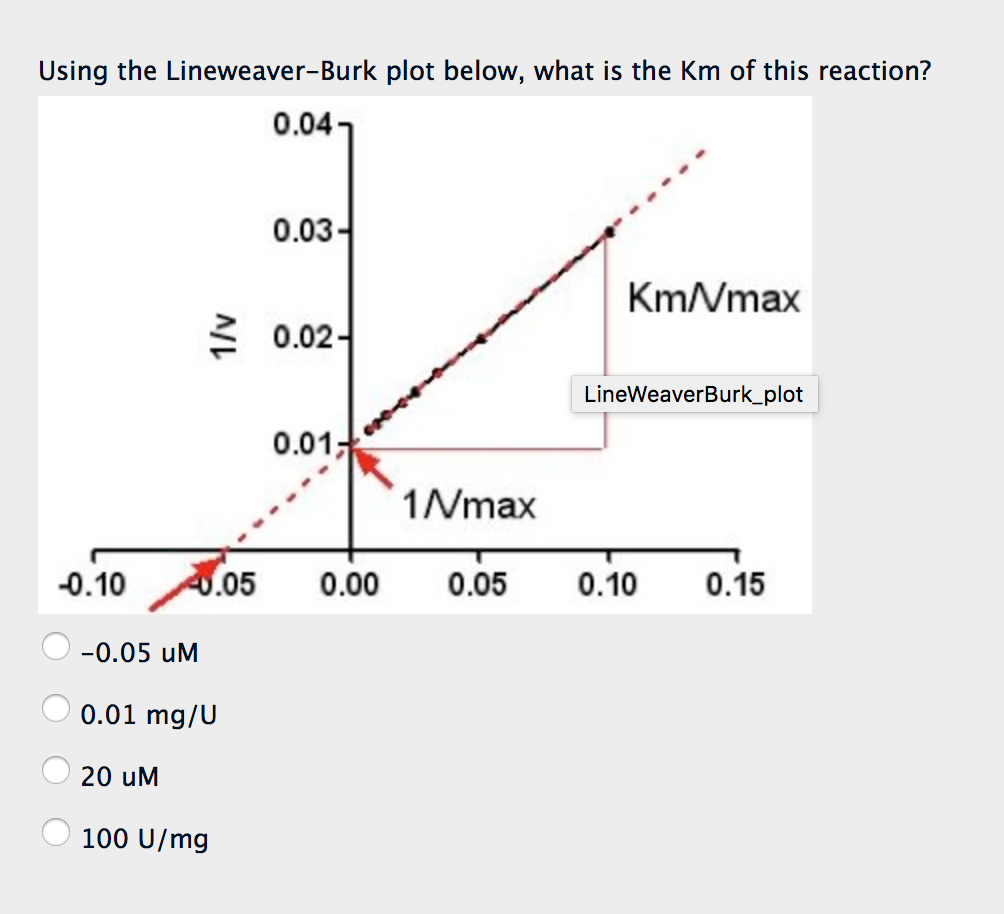
Solved Using the LineweaverBurk plot below, what is the Km
Lineweaver-Burk analysis is one method of linearizing substrate-velocity data so as to determine the kinetic constants Km and Vmax. One creates a secondary, reciprocal plot: 1/velocity vs. 1/[substrate].
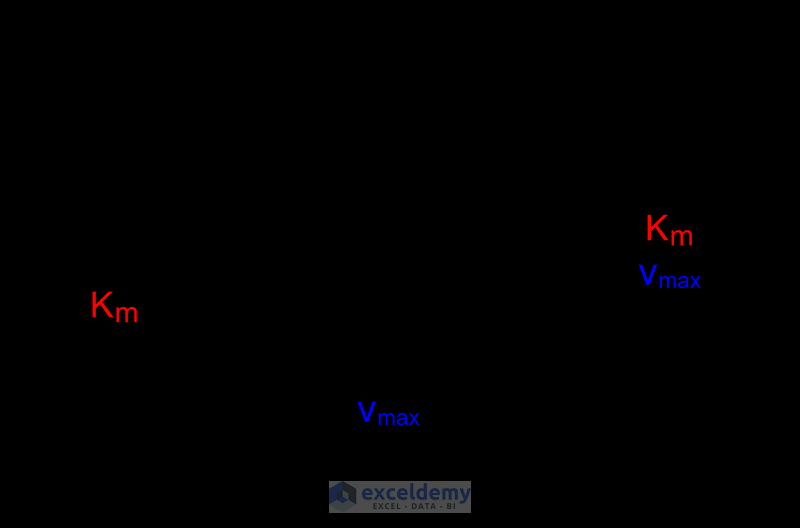
How to Make a Lineweaver Burk Plot in Excel (with Easy Steps)
The Lineweaver-Burk plot was widely used to determine important terms in enzyme kinetics, such as \(K_m\) and \(V_{max}\), before the wide availability of powerful computers and non-linear regression software. The y-intercept of such a graph is equivalent to the inverse of \(V_{max}\); the x-intercept of the graph represents \(−1/K_m\)..

Lineweaverburk plot of ACE inhibition by different concentrations of... Download Scientific
The Lineweaver-Burk reciprocal plot presents some problems due to the unequal weighting of errors as illustrated in Figure 1. Figures 1A, B and C show the same data set fit by nonlinear regression to a hyperbola (Figure 1A) compared to fits derived by linear regression using a Lineweaver-Burk plot (Figure 1B) and an Eadie-Hofstee plot (Figure 1C).

(A) LineweaverBurk plot for the inhibition of eeAChE(A) and eqBChE (B)... Download Scientific
The Lineweaver Burk plot is a graphical representation of enzyme kinetics. The x-axis is the reciprocal of the substrate concentration, or 1 / [S], and the y-axis is the reciprocal of the reaction velocity, or 1 / V. In this way, the Lineweaver Burk plot is often also called a double reciprocal plot.

LineweaverBurk Plot against substrate concentration 2 mM to 10 mM... Download Scientific Diagram
In biochemistry, the Lineweaver-Burk plot (or double reciprocal plot) is a graphical representation of the Michaelis-Menten equation of enzyme kinetics, described by Hans Lineweaver and Dean Burk in 1934. [1]

LineweaverBurk plot for determining of K M and V max (casein as... Download Scientific Diagram
The Lineweaver-Burk plot allows us to determine the Vmax and Km values of an enzyme. Vmax represents the maximum velocity of the reaction, while Km represents the substrate concentration at which the reaction velocity is half of Vmax. These parameters provide valuable insights into the enzyme's efficiency and affinity for its substrate.

The LineweaverBurk plot, representing reciprocal of initial enzyme... Download Scientific Diagram
Michaelis-Menten Graphs, Lineweaver-Burk Plots, and Reaction Schemes: Investigating Introductory Biochemistry Students' Conceptions of Representations in Enzyme Kinetics * , Nicholas P. Hux , Sven J. Philips , and Marcy H. Towns Cite this: J. Chem. Educ. 2019, 96, 9, 1833-1845 Publication Date: August 6, 2019